Revolutionizing Cancer Treatment: The Emergence of Radiopharmaceuticals
In the ever-evolving landscape of cancer treatment, the past two decades have witnessed a profound shift with the advent of targeted therapies and immunotherapies. While these innovative approaches have revolutionized cancer care, traditional treatments such as surgery, chemotherapy, and radiation therapy continue to form the cornerstone of cancer management for most patients.
Radiation therapy, employed for over a century, still plays a pivotal role in cancer treatment, with approximately half of all cancer patients undergoing it at some stage. However, until recently, radiation therapy involved directing beams of radiation externally to eradicate tumors within the body, resulting in collateral damage to surrounding healthy tissues.
Enter radiopharmaceuticals – a groundbreaking class of drugs designed to deliver radiation therapy precisely to cancer cells. In the last few years, a surge in research and clinical trials has explored the potential of these drugs to minimize both short and long-term side effects while effectively targeting minuscule cancer cell deposits throughout the body.
Charles Kunos, M.D., Ph.D., from NCI’s Cancer Therapy Evaluation Program, acknowledges the transformative potential of radiopharmaceuticals, stating, “I think they’re going to transform radiation oncology in the next 10 to 15 years.”
Natural Affinity Unleashed: A Historical Perspective
The concept of delivering radiation directly to cells isn’t entirely new. Radioactive iodine, utilized since the 1940s to treat certain types of thyroid cancer, exemplifies this approach. Exploiting the natural affinity of iodine for thyroid cells, this treatment selectively eliminates residual cancer cells post-thyroid surgery.
A similar principle underlies drugs like radium 223 dichloride (Xofigo), approved in 2013 for treating metastatic prostate cancer that has spread to bones. Radium, resembling calcium, integrates into areas with high bone turnover, effectively targeting cancer cells in bones.
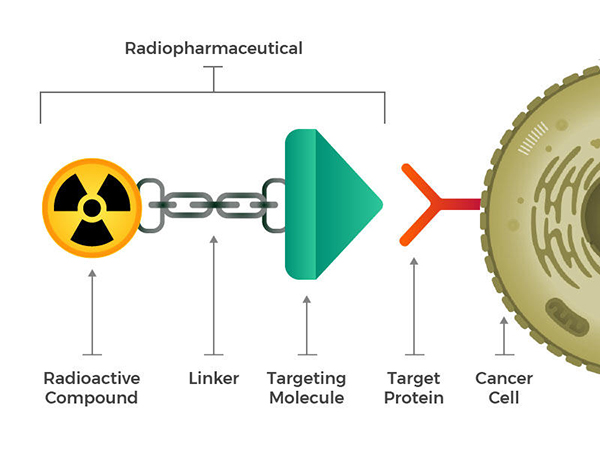
While these naturally targeted radioactive compounds paved the way, researchers aspired to engineer radiopharmaceuticals composed of a radioactive molecule, a targeting molecule recognizing cancer cells, and a linking component. These compounds, whether injected, infused, inhaled, or ingested, navigate the bloodstream and adhere to cancer cells.
The breakthrough occurred in 2018 with the FDA approval of lutetium Lu 177-dotatate (Lutathera) for neuroendocrine tumors (NETs) affecting the digestive tract. This marked a shift from repurposing existing compounds to creating radiopharmaceuticals from scratch.
Expanding Horizons: Engineering Radiopharmaceuticals for Diverse Cancers
Buoyed by this success, researchers are crafting and evaluating radiopharmaceuticals for various cancers, including melanoma, lung cancer, colorectal cancer, and leukemia. The key criterion is the presence of a targetable molecule on cancer cell surfaces and a robust blood supply for drug delivery.
Many of these innovative drugs derive from re-engineered nuclear imaging compounds, repurposing weakly radioactive molecules linked to specific cancer cell targets. Initially designed for imaging, these molecules now carry more potent radioactive isotopes, capable of not only visualizing but also eliminating cancer cells.
Prostate Cancer as a Testing Ground
Prostate cancer emerges as an early proving ground for this paradigm shift. The PSMA protein, abundant in prostate cells, becomes the focal point for radiopharmaceuticals targeting this cancer. Fusion of a PSMA-binding molecule with a radioactive compound, initially used for PET scan imaging, enables the visualization and treatment of minuscule prostate cancer deposits.
Ongoing clinical trials are investigating various PSMA-targeting radiopharmaceuticals, recognizing the potential of this approach in cases where cancer spreads throughout the body, presenting challenges for external radiation therapy.
Importantly, using imaging compounds with the same target as treatment molecules offers a preview of treatment efficacy. For example, a PET scan with the imaging compound before treatment can predict the success of the corresponding radiopharmaceutical treatment.
Paving the Way for Combination Therapies
While radiopharmaceuticals exhibit promise, they are unlikely to act as standalone tumor eradicators. For instance, lutetium Lu 177-dotatate showed significant efficacy in slowing NET growth, but combination therapies are now being explored to enhance these outcomes.
Combining radiopharmaceuticals with radiation sensitizers, drugs amplifying cancer cells’ vulnerability to radiation, is one avenue. Clinical trials, such as combining lutetium Lu 177-dotatate with the radiation sensitizer triapine, showcase the potential for improved cancer cell susceptibility.
In another realm, combining radiopharmaceuticals with PARP inhibitors is being explored. By blocking DNA repair processes, these inhibitors synergize with radiation-induced DNA damage, potentially enhancing treatment outcomes.
Additionally, researchers are investigating combinations with immunotherapies to augment their effectiveness. Radiopharmaceuticals might transform “cold” tumors, where immune cells struggle to recognize cancer, into “hot” tumors, making them responsive to immunotherapy drugs.
Combining radiopharmaceuticals with external radiation is also contemplated, provided meticulous planning ensures an overall safe radiation dose.
Challenges and Cautions in Radiopharmaceutical Development
Despite the promise, radiopharmaceuticals face challenges in widespread adoption. A shortage of physicians trained in administering these drugs hampers their personalized application. The multidisciplinary expertise required for optimal treatment planning remains limited, posing a barrier to leveraging the full potential of radiopharmaceuticals.
Long-term safety studies are imperative, and while current research suggests a low rate of late effects compared to external radiation therapy, caution and ongoing monitoring are paramount.
Collaboration and the Path Forward
Recognizing the nascent stage of radiopharmaceuticals, NCI launched the Radiopharmaceutical Development Initiative (RDI) in 2019. The initiative aims to expedite promising drugs into clinical trials, fostering collaborations between pharmaceutical companies that might not collaborate organically.
Charles Kunos emphasizes that while radiopharmaceuticals won’t replace existing radiation therapy methods, they are poised to transform how radiation is utilized. As the field continues to evolve, collaborative efforts, ongoing research, and the application of radiopharmaceuticals in combination therapies hold the promise of reshaping the future of cancer treatment.